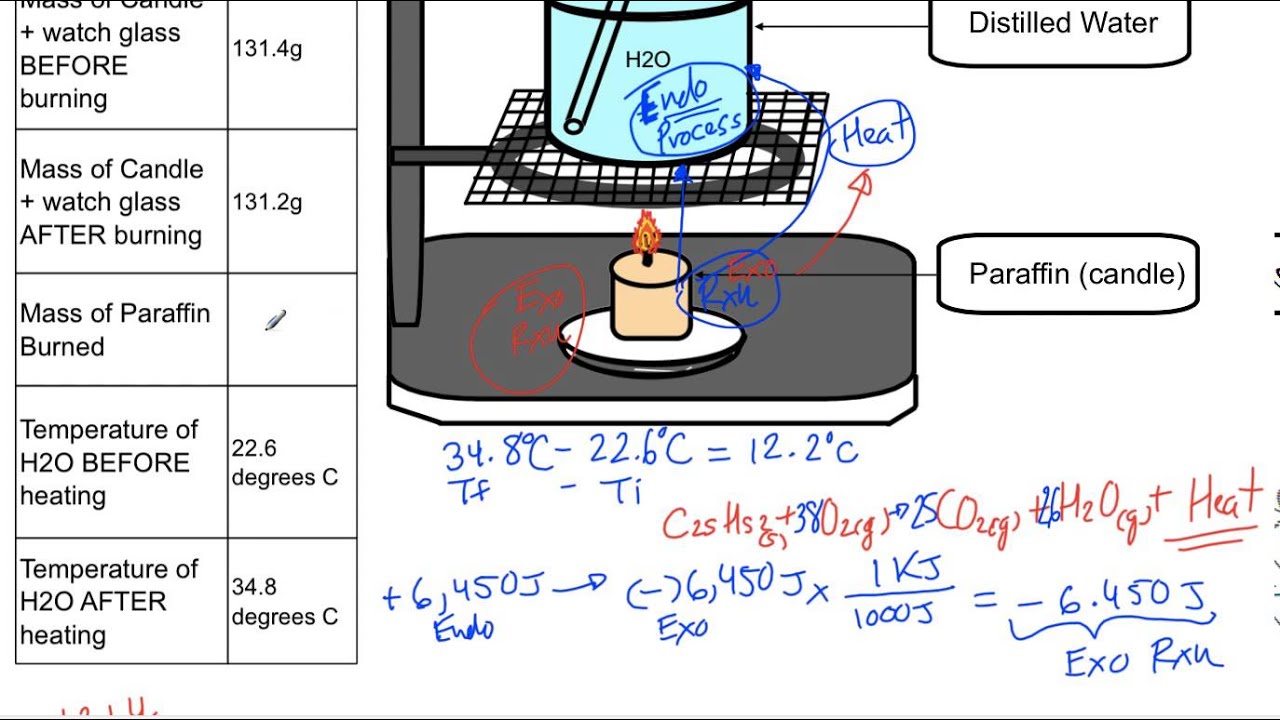
Heat Of Combustion Of A Candle Lab Report . Web standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. Alcohols burn to produce co2 and h2o. How can we determine the heat of combustion for a substance? 1) weigh the candle and the index. Some of the energy remains in the. Web calculate heat energies, using both specific heat and heat of combustion. Wax(s) + o 2 (g) → co 2 (g) + hoh(g) (equation 1) The amount of heat released is dependent on the bonds being broken and. Candle wax is a hydrocarbon (ca c 25 h 52). Web in this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o 2 (g). Validate the law of conservation of energy. Mass your candle to the nearest. Web energy is released as the bonds in the candle wax molecules and oxygen molecules break.
from www.youtube.com
Web energy is released as the bonds in the candle wax molecules and oxygen molecules break. Web calculate heat energies, using both specific heat and heat of combustion. The amount of heat released is dependent on the bonds being broken and. Some of the energy remains in the. How can we determine the heat of combustion for a substance? Candle wax is a hydrocarbon (ca c 25 h 52). Web in this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o 2 (g). 1) weigh the candle and the index. Wax(s) + o 2 (g) → co 2 (g) + hoh(g) (equation 1) Alcohols burn to produce co2 and h2o.
Enthalpy of Combustion Lab Explained YouTube
Heat Of Combustion Of A Candle Lab Report Candle wax is a hydrocarbon (ca c 25 h 52). Some of the energy remains in the. Web calculate heat energies, using both specific heat and heat of combustion. The amount of heat released is dependent on the bonds being broken and. Alcohols burn to produce co2 and h2o. How can we determine the heat of combustion for a substance? Web standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. Validate the law of conservation of energy. Web energy is released as the bonds in the candle wax molecules and oxygen molecules break. 1) weigh the candle and the index. Mass your candle to the nearest. Web in this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o 2 (g). Candle wax is a hydrocarbon (ca c 25 h 52). Wax(s) + o 2 (g) → co 2 (g) + hoh(g) (equation 1)
From www.studocu.com
Heat of Combustion Lab Report HEAT OF COMBUSTION EXPERIMENT In this Heat Of Combustion Of A Candle Lab Report Wax(s) + o 2 (g) → co 2 (g) + hoh(g) (equation 1) Validate the law of conservation of energy. The amount of heat released is dependent on the bonds being broken and. Alcohols burn to produce co2 and h2o. Web calculate heat energies, using both specific heat and heat of combustion. Candle wax is a hydrocarbon (ca c 25. Heat Of Combustion Of A Candle Lab Report.
From ar.inspiredpencil.com
Heat Of Combustion Lab Heat Of Combustion Of A Candle Lab Report The amount of heat released is dependent on the bonds being broken and. Mass your candle to the nearest. Web energy is released as the bonds in the candle wax molecules and oxygen molecules break. Wax(s) + o 2 (g) → co 2 (g) + hoh(g) (equation 1) Web standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when. Heat Of Combustion Of A Candle Lab Report.
From www.teachoo.com
Structure of a Candle Flame 3 Zones and their hotness Teachoo Heat Of Combustion Of A Candle Lab Report How can we determine the heat of combustion for a substance? Web calculate heat energies, using both specific heat and heat of combustion. Web in this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o 2 (g).. Heat Of Combustion Of A Candle Lab Report.
From www.chegg.com
Solved Heat of Combustion Mg with LabQuest In this Heat Of Combustion Of A Candle Lab Report Some of the energy remains in the. Web energy is released as the bonds in the candle wax molecules and oxygen molecules break. Web in this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o 2 (g).. Heat Of Combustion Of A Candle Lab Report.
From www.chegg.com
Solved Experiment06 The goal of this experiment is to Heat Of Combustion Of A Candle Lab Report How can we determine the heat of combustion for a substance? Mass your candle to the nearest. Some of the energy remains in the. Alcohols burn to produce co2 and h2o. Wax(s) + o 2 (g) → co 2 (g) + hoh(g) (equation 1) Validate the law of conservation of energy. Candle wax is a hydrocarbon (ca c 25 h. Heat Of Combustion Of A Candle Lab Report.
From design.udlvirtual.edu.pe
What Are The Different Parts Of Candle Flame Explain Using Labelled Heat Of Combustion Of A Candle Lab Report Some of the energy remains in the. Web in this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o 2 (g). 1) weigh the candle and the index. Web energy is released as the bonds in the. Heat Of Combustion Of A Candle Lab Report.
From www.scribd.com
Heat of Combustion of Candle Wax Combustion Oxygen Heat Of Combustion Of A Candle Lab Report Validate the law of conservation of energy. Web energy is released as the bonds in the candle wax molecules and oxygen molecules break. Alcohols burn to produce co2 and h2o. The amount of heat released is dependent on the bonds being broken and. Candle wax is a hydrocarbon (ca c 25 h 52). 1) weigh the candle and the index.. Heat Of Combustion Of A Candle Lab Report.
From mekelas.blogspot.com
Combustion Experiment With Candle / function Oxygen In the Combustion Heat Of Combustion Of A Candle Lab Report Web calculate heat energies, using both specific heat and heat of combustion. Web energy is released as the bonds in the candle wax molecules and oxygen molecules break. 1) weigh the candle and the index. Web standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. Web in this investigation, students will. Heat Of Combustion Of A Candle Lab Report.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID5861596 Heat Of Combustion Of A Candle Lab Report Web in this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o 2 (g). 1) weigh the candle and the index. Mass your candle to the nearest. Web energy is released as the bonds in the candle. Heat Of Combustion Of A Candle Lab Report.
From www.vedantu.com
What is the Scientific Reason Behind the Heat of Combustion? Heat Of Combustion Of A Candle Lab Report Alcohols burn to produce co2 and h2o. Web in this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o 2 (g). Validate the law of conservation of energy. Web energy is released as the bonds in the. Heat Of Combustion Of A Candle Lab Report.
From studylib.net
CHEMISTRY LAB HEAT OF COMBUSTION AHSHEMS Heat Of Combustion Of A Candle Lab Report Web in this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o 2 (g). Web calculate heat energies, using both specific heat and heat of combustion. The amount of heat released is dependent on the bonds being. Heat Of Combustion Of A Candle Lab Report.
From www.scribd.com
Measuring the Enthalpy of Combustion of Ethanol IB Chemistry SL Lab Heat Of Combustion Of A Candle Lab Report Alcohols burn to produce co2 and h2o. Candle wax is a hydrocarbon (ca c 25 h 52). Validate the law of conservation of energy. Web standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. Wax(s) + o 2 (g) → co 2 (g) + hoh(g) (equation 1) Web calculate heat energies,. Heat Of Combustion Of A Candle Lab Report.
From www.studocu.com
Candle Experiment Lab Report Doratti 1 Chemistry 217 Laboratory Heat Of Combustion Of A Candle Lab Report Mass your candle to the nearest. 1) weigh the candle and the index. Some of the energy remains in the. Candle wax is a hydrocarbon (ca c 25 h 52). Web energy is released as the bonds in the candle wax molecules and oxygen molecules break. The amount of heat released is dependent on the bonds being broken and. Web. Heat Of Combustion Of A Candle Lab Report.
From ar.inspiredpencil.com
Heat Of Combustion Lab Heat Of Combustion Of A Candle Lab Report Wax(s) + o 2 (g) → co 2 (g) + hoh(g) (equation 1) Web calculate heat energies, using both specific heat and heat of combustion. Web energy is released as the bonds in the candle wax molecules and oxygen molecules break. 1) weigh the candle and the index. Web standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when. Heat Of Combustion Of A Candle Lab Report.
From www.studocu.com
Lab report standard enthalpy of combustion Question Summary The Heat Of Combustion Of A Candle Lab Report The amount of heat released is dependent on the bonds being broken and. How can we determine the heat of combustion for a substance? Web in this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o 2. Heat Of Combustion Of A Candle Lab Report.
From mekelas.blogspot.com
Combustion Experiment With Candle / function Oxygen In the Combustion Heat Of Combustion Of A Candle Lab Report The amount of heat released is dependent on the bonds being broken and. Wax(s) + o 2 (g) → co 2 (g) + hoh(g) (equation 1) Web standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. Web calculate heat energies, using both specific heat and heat of combustion. Mass your candle. Heat Of Combustion Of A Candle Lab Report.
From www.chegg.com
Solved Experiment06 The goal of this experiment is to Heat Of Combustion Of A Candle Lab Report 1) weigh the candle and the index. Web calculate heat energies, using both specific heat and heat of combustion. Web in this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o 2 (g). Validate the law of. Heat Of Combustion Of A Candle Lab Report.
From www.youtube.com
Unit 5 Lesson 10 Lab Heat of Combustion YouTube Heat Of Combustion Of A Candle Lab Report Alcohols burn to produce co2 and h2o. Web calculate heat energies, using both specific heat and heat of combustion. Web standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. How can we determine the heat of combustion for a substance? Web energy is released as the bonds in the candle wax. Heat Of Combustion Of A Candle Lab Report.